Assistant Professor Rakrudee Sarthima, researcher from Department of Chemistry, Faculty of Science, Mahasarakham University, discovered protein extract with potent α-amylase inhibitory activity
Alpha-Amylase Enzyme (alpha-amylase) is found in saliva and in the small intestine of humans. It is an enzyme that accelerates the digestion of starch into maltose, which is then further digested until glucose is absorbed by the body into the bloodstream. If the alpha-amylase enzyme is inhibited, it will slow down the conversion of starch into sugar. Therefore, it can control the sugar level in the bloodstream. An example of an alpha-amylase inhibitor is Acarbose (Prandase®, Precose®), a drug used to control blood sugar or weight and is used in people with diabetes (Yamagishi et al., 2009). One interesting alpha-amylase inhibitor, white bean extract, has been shown to be effective in reducing weight in subjects (Barrett et al., 2011). White bean extract, therefore, has been mixed in many products.

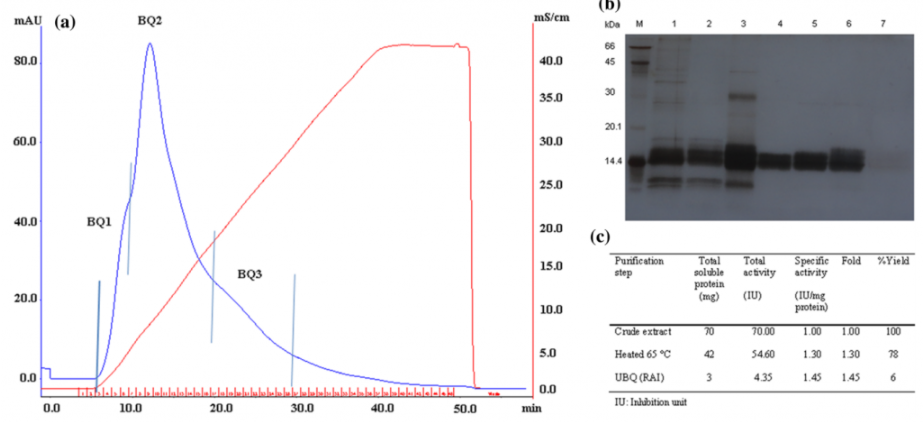
Asst. Prof. Rakrudee Sarthima and colleagues conducted the research on the topic “Glutinous rice (Oryza sativa L.) protein extract with potent α-amylase inhibitory activity” which was published in the Journal of Food Science and Technology, vol. 57, page 3157-3163 in 2020. This research screened for α-amylase inhibitory activity of twenties-five varieties of Thai indigenous rice seeds. Based on specific inhibition, crude protein of var. Gai Ngaw (Gs. No. 13719) was selected for purification. The unbound proteins of the Q-Sepharose column named partially purified rice α-amylase inhibitor (RAI) revealed MW of approximately 14.4 kDa. The RAI was stable at pH 4 to 7 and heat stable up to 80 °C. The RAI had IC50 of 15.92 ± 1.08 µg/ml. The double reciprocal plot implied a mixed-type inhibitor. The Dixon and Cornish-Bowden plots were used to estimate Ki and αKi. This suggested Thai indigenous rice seeds could potentially be developed as a food supplement for blood sugar and weight controls.
Reference
Sarnthima, R., Khammuang, S. & Joompang, A. Glutinous rice (Oryza sativa L.) protein extract with potent α-amylase inhibitory activity. J Food Sci Technol 57, 3157–3163 (2020). https://doi.org/10.1007/s13197-020-04560-w
Barrett, M.L., Udani, J.K. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): A review of clinical studies on weight loss and glycemic control. Nutr J 10, 24 (2011). https://doi.org/10.1186/1475-2891-10-24
Yamagishi S, Matsui T, Ueda S, Fukami K, Okuda S: Clinical utility of acarbose, an alpha-glucosidase inhibitor in cardiometabolic disorders. Curr Drug Metab. 2009, 10: 159-163. Doi: 10.2174/138920009787522133